Overview of Tablet Testing Instruments in India

Tablet testing is a crucial aspect of pharmaceutical manufacturing to ensure the quality, safety, and efficacy of oral dosage forms. In India, the pharmaceutical industry adheres to global standards, with the United States Pharmacopeia (USP) providing guidelines and procedures for various tests. In this comprehensive guide, we will delve into the procedures and criteria outlined […]
Antibiotic Potency Test – Diffusion (Cylinder-Plate) Method USP, BP/EP, JP
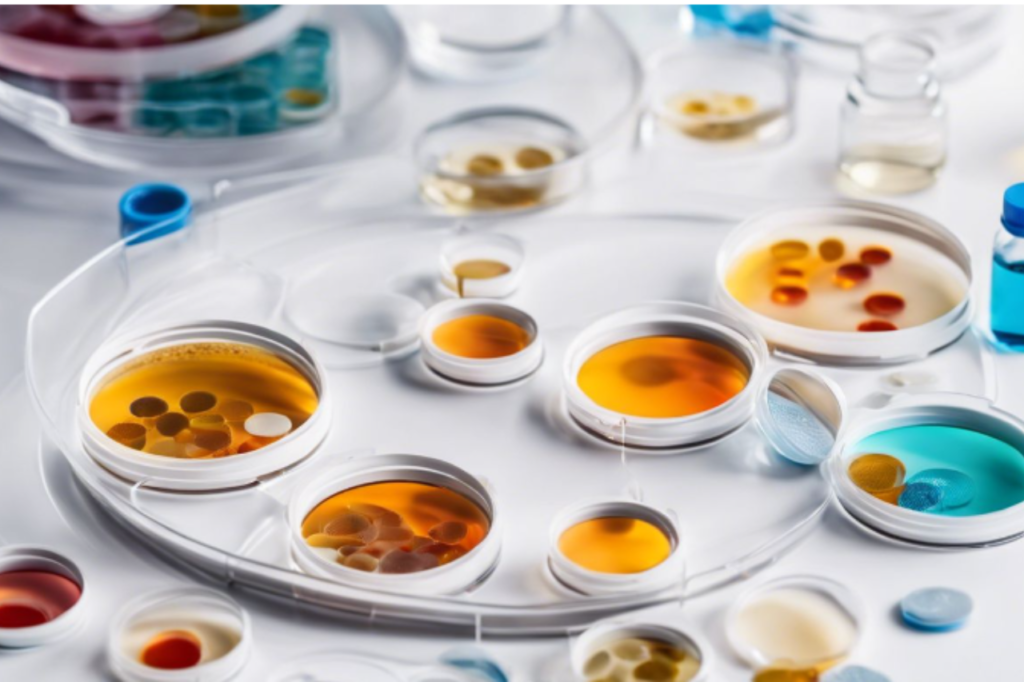
Understanding the strength of an antibiotic is a vital step in fighting infections effectively. Without precise potency measurement, treatments can falter, resistance can grow, and patient outcomes can worsen. Drawing upon years of experience within pharmaceutical microbiology, I bring to light the critical aspects of antibiotic potency testing – a keystone in ensuring medications work […]
Antibiotic Zone Reader

In the fight against antibiotic resistance, accurate measurement of antimicrobial activity is crucial. The Antibiotic Zone Reader is a sophisticated instrument that revolutionizes how we perform antibiotic potency testing. With its ability to measure inhibition zone diameters from 0 to 35 mm with a high level of precision, this device is essential in pharmaceutical industries, […]

