Role of Tablet Dissolution Testing in Drug Development and Bioavailability Studies
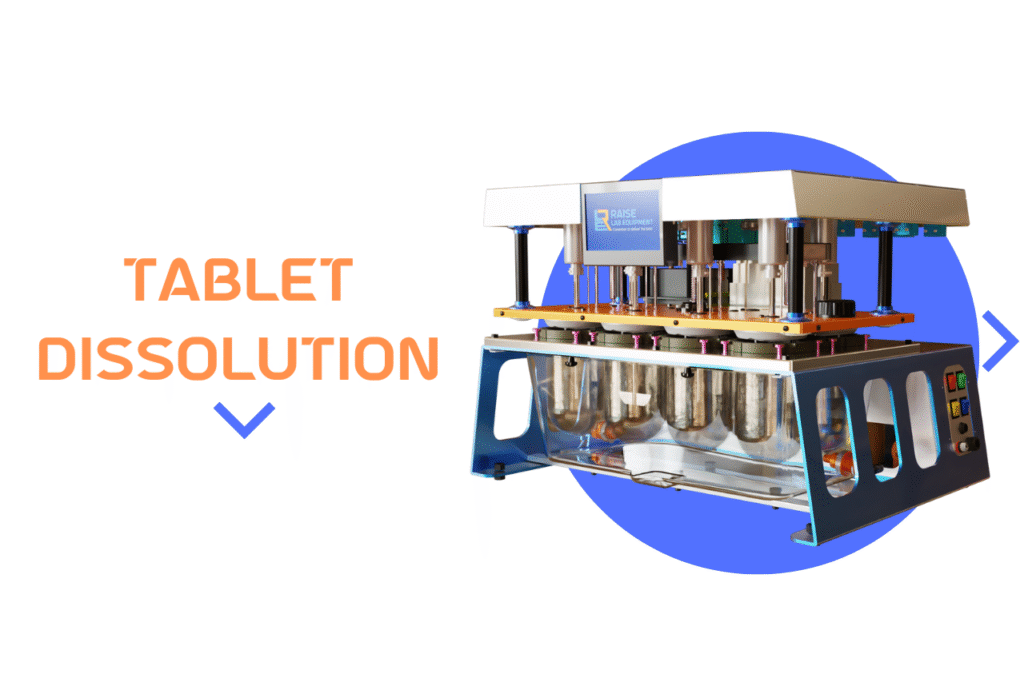
Tablet dissolution testing is a cornerstone analytical technique in pharmaceutical drug development and bioavailability studies. It measures the rate and extent at which the active drug substance in a solid oral dosage form (tablet) dissolves into a specified medium under controlled conditions that simulate the physiological environment of the gastrointestinal (GI) tract. This testing profoundly […]
Standard Operating Procedure for Tap Density Apparatus as per USP Standards

Tap Density Apparatus: In pharmaceutical manufacturing and formulation development, the physical properties of powders play a critical role in process performance, flow behavior, and final product quality. Among these, tapped density is one of the most essential parameters, offering insight into the packing and compressibility characteristics of powder materials. To ensure valid and reproducible results, […]
Understanding the Difference Between Manual, Offline Sampling, and Online Sampling Dissolution Testers

Understanding the Difference Between Manual, Offline Sampling, and Online Sampling Dissolution Testers Dissolution testing is a critical analytical technique in the pharmaceutical industry, used to measure the rate and extent to which the active pharmaceutical ingredient (API) is released from a solid dosage form (like tablets or capsules) into a solution. This process plays a […]
Automated Tablet Disintegration Testing: Features, Benefits, and Compliance

Automated Tablet Disintegration Testing In the pharmaceutical industry, ensuring the quality and efficacy of oral dosage forms is paramount. Automated tablet disintegration testers play a crucial role in this process by providing precise and reliable assessments of how tablets disintegrate under specified conditions. These instruments are designed to comply with international standards, including USP chapters […]
Validation of Dissolution Test Apparatus: Guaranteeing Precision in Pharmaceutical Analysis

Validation of Dissolution Test Apparatus In the pharmaceutical industry, the accuracy and reliability of analytical testing are paramount. Among the crucial tests conducted is dissolution testing, which evaluates the rate at which an active pharmaceutical ingredient (API) is released from a solid dosage form. The integrity of this testing hinges significantly on the performance of […]
Dissolution Tester Calibration and Validation Process

Dissolution Tester Calibration and Validation Process Introduction: Dissolution tester is a crucial quality control procedure in the pharmaceutical industry, ensuring the consistent release and bioavailability of active pharmaceutical ingredients (APIs) in various dosage forms. To obtain accurate and reproducible results, dissolution testers must undergo regular calibration and validation. These processes verify that the equipment functions […]
Dissolution Tester USP-1, 2, 5, 6 and Intrinsic Methods

Dissolution Tester USP-1, 2, 5, 6 Methods Introduction: Dissolution Tester USP plays a critical role in the pharmaceutical industry by evaluating the rate at which an active pharmaceutical ingredient (API) dissolves in a given medium. It is an essential quality control test used to ensure consistency, efficacy, and bioavailability of oral solid dosage forms such […]
How to do calibration to Tablet Hardness Tester and what parameters need to be tested

How to do calibration to Tablet Hardness Tester Introduction Calibration of a Tablet Hardness Tester is a critical process in the pharmaceutical industry to ensure accurate and consistent measurements. Hardness testers measure the force required to break a tablet under controlled conditions, ensuring quality control and compliance with regulatory standards. Regular calibration helps maintain the […]
How to do Calibration to Tablet Friability and what are parameters need to be tested

Calibration to Tablet Friability Introduction Tablet friability is a critical quality control parameter in the pharmaceutical industry that assesses the mechanical strength of tablets during handling, packaging, and transportation. The friability test evaluates a tablet’s ability to withstand abrasion and breakage under stress. To ensure accurate and reliable results, the friability tester must be periodically […]
Tablet Disintegrant Ability: Sensitivity of Super disintegrants to Temperature and Compaction Pressure

Tablet Disintegrant Ability Tablet disintegration ability is a critical factor in determining the bioavailability of a drug. For a drug to be effectively absorbed by the body, the tablet must break down in the gastrointestinal tract, releasing the active ingredients. Disintegration refers to the breakdown of the tablet into smaller particles when it comes into […]

