Tap Density Apparatus:
In pharmaceutical manufacturing and formulation development, the physical properties of powders play a critical role in process performance, flow behavior, and final product quality. Among these, tapped density is one of the most essential parameters, offering insight into the packing and compressibility characteristics of powder materials.
To ensure valid and reproducible results, testing must comply with pharmacopeial procedures, particularly those described under USP-I and USP-II standards. This article explains the complete Standard Operating Procedure (SOP) for conducting the Tapped Density Test using the TD‑2 Tapped Density Apparatus from Raise Lab Equipment, a reliable, microcontroller-based instrument engineered for precision and regulatory compliance.
Importance of Tapped Density Testing
Tapped density testing determines the volume reduction of a powdered material subjected to controlled mechanical tapping. The test simulates the vibration and compaction that powders experience during processing, transportation, and packaging. Measuring the tapped density enables the calculation of key properties such as Compressibility Index and Hausner Ratio, which together help assess the powder’s flow characteristics and uniformity. Proper measurement supports consistent blend uniformity, accurate capsule filling, and stable tablet formation, core objectives of pharmaceutical quality assurance.
Raise Lab Equipment’s TD‑2 Apparatus is specifically designed to meet strict pharmacopeial requirements, ensuring accuracy, repeatability, and ease of operation. The unit’s stepper motor‑driven technology provides consistent drop height and tapping frequency, minimizing variation from mechanical wear over time. With a single-station, space-efficient design, the instrument fits well into modern analytical laboratories.
1. Key Technical Specifications and USP Methods
The TD‑2 Tapped Density Apparatus supports two standard test modes: USP‑I and USP‑II, along with a Custom Method for user-defined parameters. In compliance with both pharmacopeial models, the TD‑2 performs controlled tap sequences with specific drop heights and tapping numbers, depending on the selected method.
| Parameter | USP-I | USP-II |
| Drops per Minute (DPM) | 300 ± 15 | 250 ± 15 |
| Drop Height | 14 mm ± 2.0 | 3 mm ± 0.2 |
| Tapping Counts (Steps 1–3) | 10, 500, 1250 | 10, 500, 1250 |
If the difference between consecutive step volumes at Step 3 exceeds two percent, the instrument can automatically proceed to Step 4 or Step 5, repeating 1250 taps until the variation is within the 2 % acceptance limit. This ensures the test concludes only when the powder achieves consistent packing density.
2. Instrument Setup and Preparation
Proper setup is vital for the reliability of results. Follow these steps before initiating the test:
- Placement and Power:
Position the TD‑2 apparatus on a sturdy, level surface. Connect both the main power cord and printer cable to their respective rear sockets. - Cylinder Preparation:
The instrument comes equipped with 250 mL and 100 mL Class A glass cylinders and corresponding holders. Class A cylinders may also be used for higher precision. - Mounting the Cylinder:
Secure the cylinder onto its holder, ensuring proper engagement with the locating pin before starting any test. - Adaptor Usage:
- For USP‑I, remove the Derlin adaptor and mount the holder directly.
- For USP‑II, use the SS adaptor before fixing the holder. Either cylinder may be used for both methods, based on sample volume.
- For USP‑I, remove the Derlin adaptor and mount the holder directly.
- Sample Preparation:
Weigh approximately 100 g of the test powder and pass it through a 1.00 mm (No. 18) screen to eliminate clumps and ensure particle uniformity. Transfer the powder to a dry measuring cylinder and carefully level it without compressing. Record the initial apparent volume (V₀) to the nearest unit.
3. Conducting the Tapped Density Test (USP Method)
Once the apparatus and sample are ready, proceed with the test under USP guidelines.
A. Initial Login and Configuration
Switch on the instrument using the rear power key. After the welcome screen displays the manufacturer, model, and version, press ‘Start’ to continue.
Select your access level per CFR Part 11 requirements:
- ADMINISTRATOR: ‘Start’ key
- SUPERVISER: ‘Stop’ key
- USER: ‘Enter’ key
Navigate to TEST SETTINGS using the left arrow key.
Set the Test Method (USP‑I or USP‑II) and Cylinder Type (250 mL or 100 mL).
Define tapping counts for Steps 1, 2, and 3 as 10, 500, 1250, respectively.
Finally, input the initial volume (V₀) by pressing the corresponding keys.
B. Executing the Test
From the main menu, press Start and enter the Batch No, Product Name, and Operator Name.
Choose the USP method, then input the sample weight (in grams) and initial volume (V₀) in milliliters.
The test begins with Step 1, where the instrument executes the first set of taps according to the selected USP parameters.
C. Volume Measurement and Continuation
After Step 1 completes, press Stop, measure the sample volume, and enter the reading. The system calculates the difference. Press Start to proceed to Step 2, repeating the process for Steps 2 and 3. If the volume difference between any two steps exceeds 2 %, continue to additional steps (Step 4 or 5) until the deviation falls within the acceptance range.
D. Result Calculation and Reporting
Upon completion, the display shows Tapped Density (W/Vf), Initial Bulk Density, Compressibility Index, and Hausner Ratio. A summarized test report can be printed instantly by pressing ‘Start’ when prompted.
4. Formulae Used
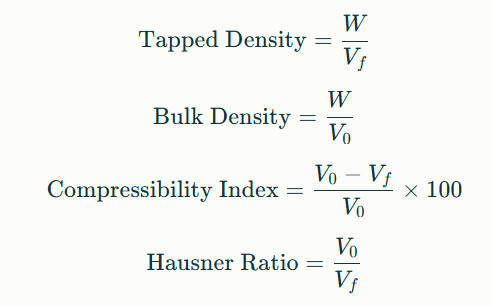
Where WWW represents the powder weight (g), V0V_0V0 the initial volume, and VfV_fVf the final tapped volume.
5. Acceptance and Documentation
According to USP criteria, the tapping process concludes once the successive volume change is ≤ 2 mL or 2 %. Record all results in the Tapped Density Worksheet or capture them electronically via the TD‑2 integrated interface. Attach printouts, verify values, and archive the data as part of the batch documentation approved by Quality Assurance.
Conclusion
The TD‑2 Tapped Density Apparatus from Raise Lab Equipment provides a precise, compliant, and user-friendly solution for powder density determination in both routine QC environments and R&D studies. By adhering to this SOP, laboratories can ensure reliable data consistency, maintain compliance with USP‑I and USP‑II requirements, and uphold the highest standards of pharmaceutical quality control.


